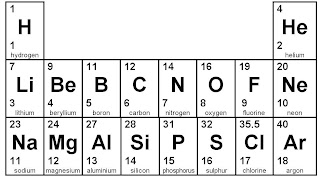
To work out where to put the electrons, start by looking at the Periodic Table. You need the bottom number to give you the number of electrons.
Let's start with ALUMINIUM. It has 13 electrons because the bottom number is 13.
We get the 13 like this:
- The first 2 go on the inside layer.
- The next 8 go on the second layer out.
- 2 and 8 make 10. That means there are 3 left for the outside layer.
Oxygen is the next example. It's bottom number on the Periodic Table is 8.
- 2 on the inside layer.
- That means that there are 6 left to go on the second layer. (It can have up to 8)
Final example is lithium. It's bottom number is 3.
- 2 on the inside layer.
- That leaves 1 on the second layer this time.



No comments:
Post a Comment