This when you mix two liquids together and end up with a solid floating in the liquid. The solid is called a precipitate.
We did it when we made the chemical that they put on film. We got a white precipitate which we filtered out. We spread the white precipiate out on the filter paper and put it on the side in the sunshine with a coin over it.
Where the sun reached it, a reaction happened and the chemical went dark grey. Under the coin there was no light and no reaction so it stayed white.
Thursday, 18 December 2008
Covalent bonds
This is where atoms share electrons to make them think that they have full outside layers. They are weak bonds, so the chemicals have low melting and boiling points.
This is chlorine.
 An odd one is diamond. Each carbon atom is joined to 4 other atoms in a huge structure that is very strong.
An odd one is diamond. Each carbon atom is joined to 4 other atoms in a huge structure that is very strong.Ionic bonding
Atoms are only "happy" when their outside layers are either completely full or completely empty. The atom on the left had 2 atoms on its outside layer.
are only "happy" when their outside layers are either completely full or completely empty. The atom on the left had 2 atoms on its outside layer.
To get a completely empty layer, it loses two electrons. Taking away 2 negative electrons makes it doubly positive (2+).
The atom on the right had only 6 electrons on its outside layer. It needs 8 to have a full layer so it gains two electrons. Gaining 2 negative electrons makes it doubly negative (2-).
Opposites attract so the (2+) and the (2-) stay together. This is an ionic bond. The bonds are very strong so ionic compounds have very high melting and boiling points.

 are only "happy" when their outside layers are either completely full or completely empty. The atom on the left had 2 atoms on its outside layer.
are only "happy" when their outside layers are either completely full or completely empty. The atom on the left had 2 atoms on its outside layer. To get a completely empty layer, it loses two electrons. Taking away 2 negative electrons makes it doubly positive (2+).
The atom on the right had only 6 electrons on its outside layer. It needs 8 to have a full layer so it gains two electrons. Gaining 2 negative electrons makes it doubly negative (2-).
Opposites attract so the (2+) and the (2-) stay together. This is an ionic bond. The bonds are very strong so ionic compounds have very high melting and boiling points.

How to work out where to put electrons
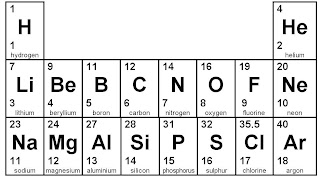
To work out where to put the electrons, start by looking at the Periodic Table. You need the bottom number to give you the number of electrons.
Let's start with ALUMINIUM. It has 13 electrons because the bottom number is 13.
We get the 13 like this:
- The first 2 go on the inside layer.
- The next 8 go on the second layer out.
- 2 and 8 make 10. That means there are 3 left for the outside layer.
Oxygen is the next example. It's bottom number on the Periodic Table is 8.
- 2 on the inside layer.
- That means that there are 6 left to go on the second layer. (It can have up to 8)
Final example is lithium. It's bottom number is 3.
- 2 on the inside layer.
- That leaves 1 on the second layer this time.
Tuesday, 16 December 2008
Electrolysis
Electolysis is where we use an electric current to split up an ionic compound that has been dissolved in water.
Our example was sodium chloride solution (salt water)
It contains H+ and OH- ions from the water.
It contains Na+ and Cl- ions from the chlorine.
Two Cl- ions lose 2 electrons at the positive electrode and turn into chlorine gas. It bleached blue litmus paper, turning it white.
The two electrons join up with two H+ ions at the negative electrode to make hydrogen gas.
Na+ and OH- are left behind. This makes sodium hydroxide which is an alkali and turns the universal indicator purple.
Our example was sodium chloride solution (salt water)
It contains H+ and OH- ions from the water.
It contains Na+ and Cl- ions from the chlorine.
Two Cl- ions lose 2 electrons at the positive electrode and turn into chlorine gas. It bleached blue litmus paper, turning it white.
The two electrons join up with two H+ ions at the negative electrode to make hydrogen gas.
Na+ and OH- are left behind. This makes sodium hydroxide which is an alkali and turns the universal indicator purple.
Subscribe to:
Comments (Atom)



